In the field of therapeutic antibody production, diversification of fed-batch strategies is flourishing in
response to the market demand. All manufacturing approaches tend to follow the generally accepted dogma
of increasing titer since it directly increases manufacturing output. While titer is influenced by the biomass
(expressed as IVCD), the culture time and the cell specific productivity (qp), we changed independently each
of these parameters to tune our process strategy towards adapted solutions to individual manufacturing
needs.
To do so, we worked separately on the increase of the IVCD as high seeding fed-batch capacity. Yet, as
intensified fed-batch may not always be possible due to limited facility operational mode, we also separately
increased the qp with the addition of specific media additives. Both strategies improved titer by 100% in 14
days relative to the standard fed-batch process with moderate and acceptable changes in product quality
attributes.
Since intensified fed-batch could rival the cell-specific productivity of a conventional fed-batch, we developed
novel hybrid strategies to either allow for acceptable seeding densities without compromising productivity, or
alternatively, to push the productivity the furthest in order to reduce timelines.
Selexis admin
Recent posts by Selexis admin

1 min read
CHO Fed-Batch Strategies To Rapidly Increase mAb Titer By 100% Without Sacrificing Product Quality
Keywords: CHO Intensified fed-batch ambr15 2022
1 min read
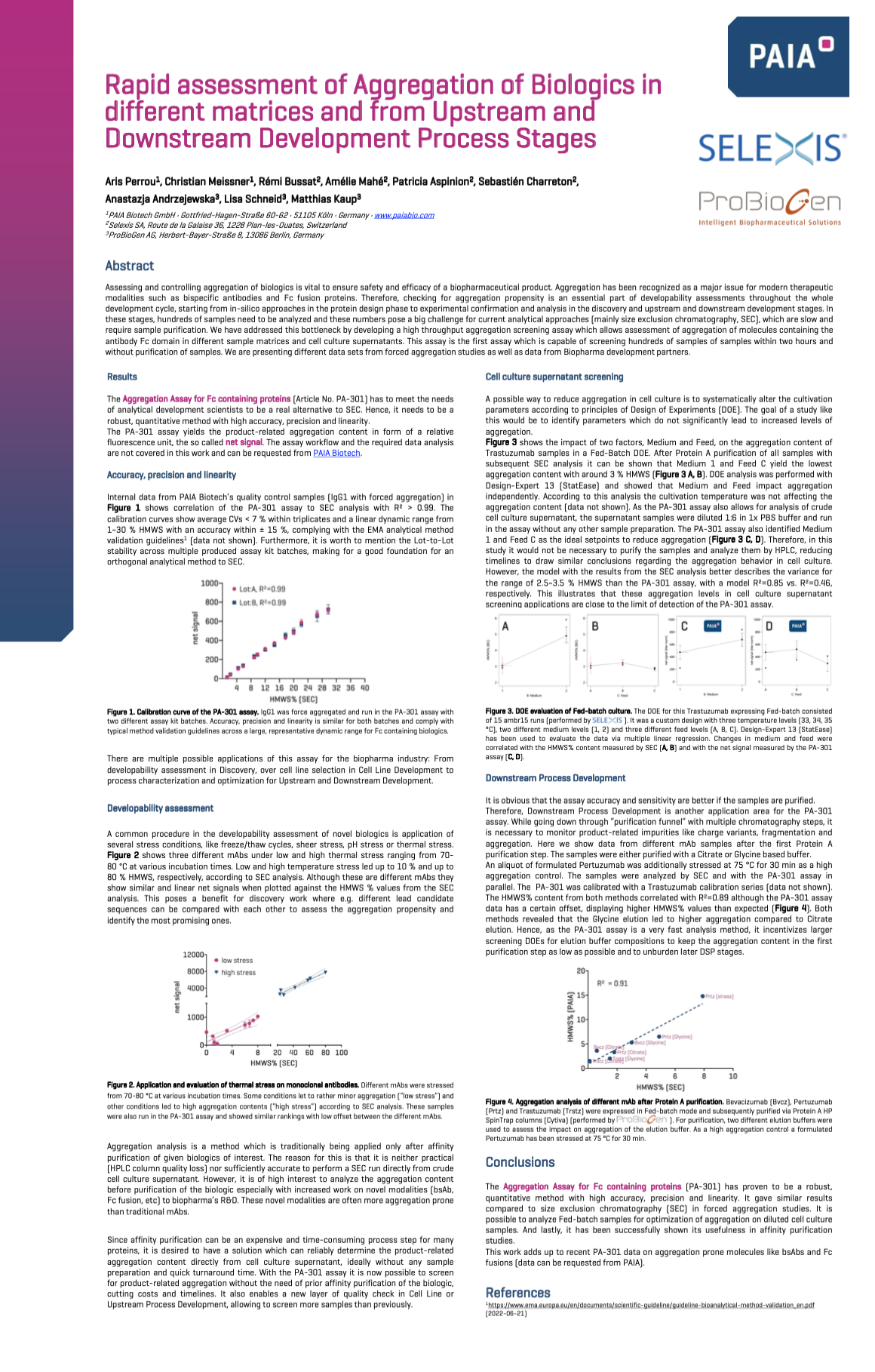
Rapid assessment of Aggregation of Biologics in Different Matrices and from Upstream and Downstream Development Process Stages
Assessing and controlling aggregation of biologics is vital to ensure the safety and efficacy of a biopharmaceutical product. In addition, aggregation has been recognized as a major issue for modern therapeutic modalities such as bispecific antibodies and Fc fusion proteins. Therefore, checking for aggregation propensity is an essential part of developability assessments throughout the development cycle, starting from in silico approaches in the protein design phase to experimental confirmation and analysis in the discovery and upstream and downstream development stages.
In these stages, hundreds of samples need to be analyzed. These numbers pose a big challenge for current analytical approaches (mainly size exclusion chromatography, SEC), which are slow and require sample purification. We have addressed this bottleneck by developing a high throughput aggregation screening assay that allows the assessment of aggregation of molecules containing the antibody Fc domain in different sample matrices and cell culture supernatants. This is the first assay capable of screening hundreds of samples within two hours and without purification of samples. We are presenting different data sets from forced aggregation studies and data from Biopharma development partners.
Keywords: research cell bank Recombinant protein production next-generation sequencing genomic analysis
1 min read
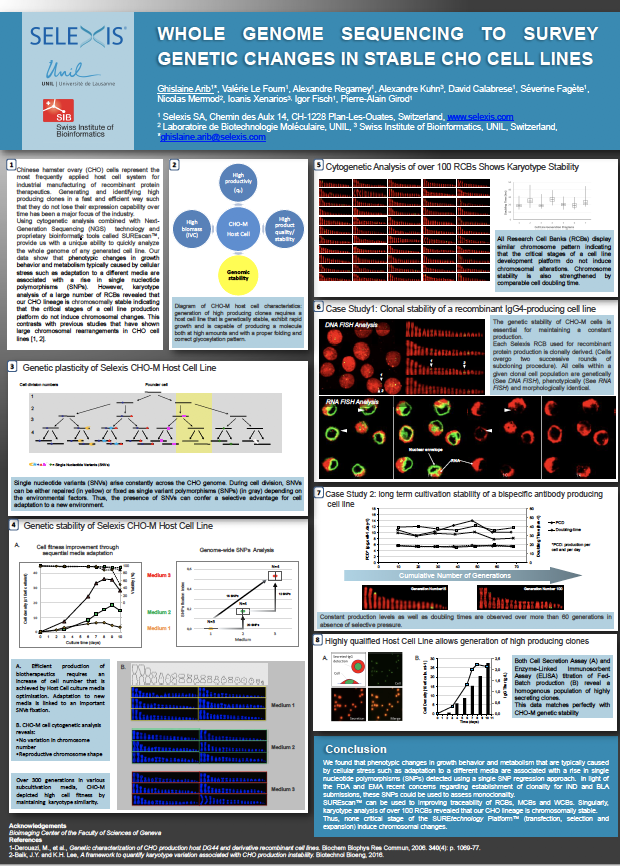
Whole Genome Sequencing to Survey Genetic Changes in Stable CHO Cell Lines
Chinese hamster ovary (CHO) cells represent the most frequently applied host cell system for industrial manufacturing of recombinant protein therapeutics. Generating and identifying high producing clones in a fast and efficient way such that they do not lose their expression capability over time has been a major focus of the industry.
Using cytogentic analysis combined with next-generation sequencing technology and proprietary bioinformatic tools called SUREscan, provide us with a unique ability to quickly analyze the whole genome of any generated cell line. Our data show that phenotypic changes in growth behavior and metabolism typically cuased by cellular stress such as adaptation to a different media are associated with a rise in single nucleotide polymorphisms (SNPs). However, karyotype analysis of a large number of research cell banks (RCBs) revealed our CHO lineage is chromosomally stable indicating that the critical stages of a call line production platform do not induce chromosomalchanges. This contrasts with previous studies that have sown large chromosomal rearrangements in CHO cell lines.
Keywords: research cell bank CHO Chinese hamster ovary next-generation sequencing SUREscan recombinant protein whole genome sequencing single nucleotide polymorphisms chromosomal rearrangements

1 min read
SELEXIS SUREscan: De-risking Cell Bank Generation with Comprehensive Genomic Analysis
Recombinant therapeutic protein production processes must guarantee a sufficiently small variability in the product quality. To keep this variability low, it is critical to run the process in a totally reproducible way. This requires controlling all cultivation parameters. The use of new analyzers, generating new data sets, besides cultivation parameters (e.g. viable cell density, metabolite concentrations) is a desirable way to innovate bioprocesses. The advent of Next-generation Sequencing (NGS) has led to the ability of using genome information to find reasons for variability. Research Cell Banks (RCBs) are not necessarily cell populations with identical genomes or single integration sites even though they arise from a single isolated cell. These mixed populations can lead to unacceptable manufacturing variability. Selexis’ SUREscan™, consisting of the detailed CHO-M genomic map and proprietary bioinformatics tools, decreases manufacturing risks by ensuring transgene integrity in RCBs and by surveying for the emergence of deleterious mutations either in the transgene sequence or in genes that are important for cell survival at a yet unknown resolution.
Keywords: research cell bank Recombinant protein production next-generation sequencing genomic analysis

1 min read
CHO Cell Library for the Selection of Improved Recombinant Therapeutic Protein Production Clones
In an effort to improve product yield of mammalian cell lines, we have previously demonstrated that our proprietary DNA elements, Selexis Genetic Elements (SGEs), increase the transcription of a given transgene1, thus boosting the overall expression of a therapeutic protein drug in mammalian cells. However, there are additional cellular bottlenecks, notably in the molecular machineries of the secretory pathways. Most importantly, mammalian cells have some limitations in their intrinsic capacity to manage high level of protein synthesis as well as folding recombinant proteins. These bottlenecks often lead to increased cellular stress and, therefore, low production rates. Our specific approach involves CHO cell line engineering. We constructed a CHO-M library based upon the CHO-M genome and transcriptome and using unique proprietary transposon vectors harboring SGE DNA elements to compensate for rate-limiting factors. This CHO-Mplus library displays a diversity of greater than 1×107 auxiliary proteins involved in secretory pathway machineries and cellular metabolism. We show that our CHO-Mplus library enabled the selection of a clonal cell line expressing 10 fold more product by comparison to standard approaches.
Keywords: Chinese hamster ovary genome CHO Cell protein production clones cellular bottlenecks CHO-Mplus Library transcriptome
1 min read
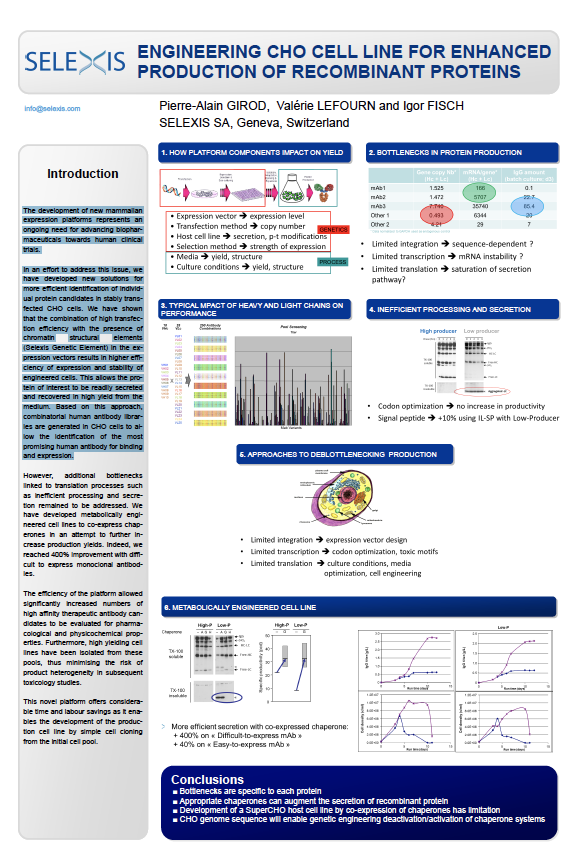
Engineering CHO Cell Lines for Enhanced Production of Recombinant Proteins
The development of new mammalian expression platforms represents an ongoing need for advancing biophar-maceuticals towards human clinical trials.
In an effort to address this issue, we have developed new solutions for more efficient identification of individ-ual protein candidates in stably trans-fected CHO cells. We have shown that the combination of high transfec-tion efficiency with the presence of chromatin structural elements (Selexis Genetic Element) in the ex-pression vectors results in higher effi-ciency of expression and stability of engineered cells. This allows the pro-tein of interest to be readily secreted and recovered in high yield from the medium. Based on this approach, combinatorial human antibody librar-ies are generated in CHO cells to al-low the identification of the most promising human antibody for binding and expression.

